Technology 
Accessible single cell
The end-to-end solution
Our solution takes you from single cell or single-nuclei suspension through library prep and sequencing and delivers immediate results via our analysis software, Trailmaker.
Products 
Products
Whole Transcriptome
Evercode WT Mini
Get started with single cell
Evercode WT
Achieve your single cell ambitions
Evercode WT Mega
A simpler way to scale your single cell projects
Evercode WT Penta
Scale single cell experiments without compromise
Coming Soon
Evercode™ FFPE
With Evercode FFPE, researchers can now see the complete molecular landscape of each cell without the constraints of predefined probe panels.
Resources 
Resources
Resources
Explore our collection of Evercode resources
Publications
Publications featuring Evercode in single cell research
Product Literature
Delve deeper into the product details
Customer Datasets
Customer datasets covering various applications and sample types
Datasets
Datasets covering various applications and sample types
Data Analysis
Trailmaker
Our user-friendly platform simplifies scRNA-seq data analysis, making it accessible and transparent for researchers.
Company 
Company
About Parse
Providing researchers single cell sequencing with unprecedented scale and ease
Upcoming Events
Conferences and in-person meetings
Blog
Customer interviews, tips and tricks, and more
Distributors
Our single cell solutions are available via a growing network of authorized distribution partners.
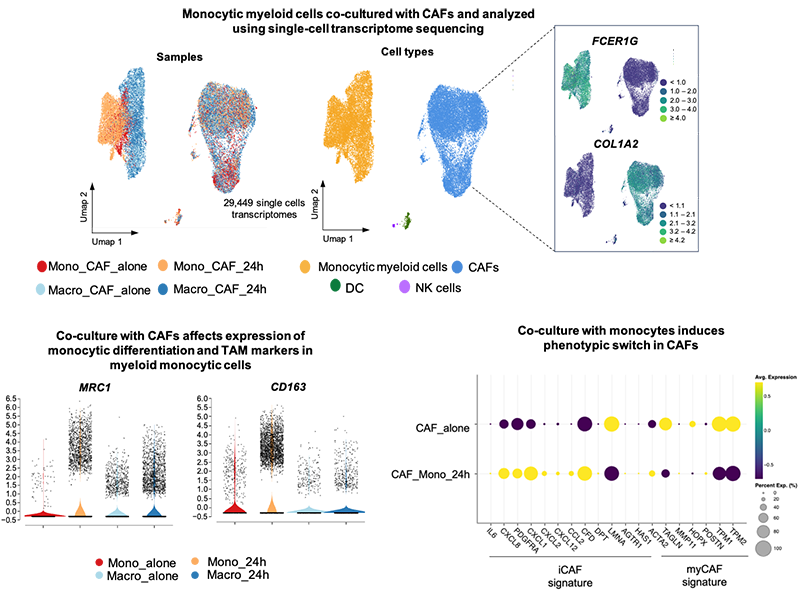
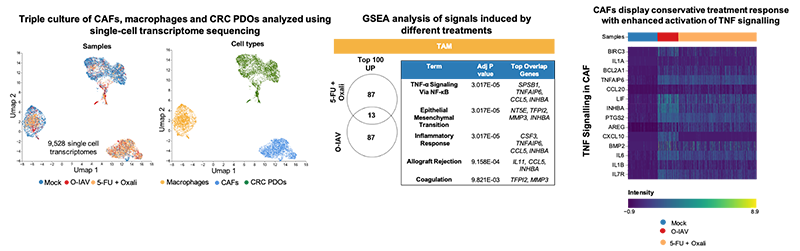
 Anna is a senior postdoctoral researcher in Dr Mattias Farlik’s lab at the Medical University of Vienna.
Anna is a senior postdoctoral researcher in Dr Mattias Farlik’s lab at the Medical University of Vienna.