The following research publications demonstrate the potential discoveries unlocked by employing alternative animal models.
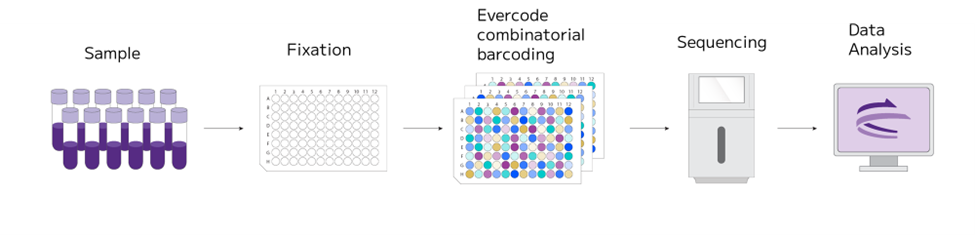
Scientists employed the Parse Evercode™ assay, a plate-based combinatorial barcoding approach. This technology, not limited by specific models, integrates a protocol for sample fixation, effectively preserving the whole transcriptome. Such integration facilitates the execution of extended or longitudinal studies, while mitigating batch effect concerns. It also allows for deferred sample processing, significantly streamlining the operational demands of long-term studies.
Small Models: Zebrafish and Drosophila Melanogaster
Zebrafish (Danio Rerio) is a valuable model organism for studying human development and disease due to its cost-effectiveness compared to other vertebrate models. Zebrafish are genetically and physiologically similar to humans, sharing 70% of their protein-coding genes. Their transparent embryos develop externally, allowing real-time observation of developmental processes. The zebrafish genome is relatively simple and amenable to genetic editing, making it ideal for investigating multigenic diseases. Zebrafish were the first vertebrate model to demonstrate efficient genome editing using CRISPR in vivo, and they are particularly useful for high-throughput drug screening.
Zebrafish enable real-time monitoring of developmental processes, from embryogenesis to organ formation, within 36 hours post-fertilization. This rapid development facilitates detailed examination of a vaccine’s impact on organ precursors.
Remarkably, zebrafish have the capacity to regenerate heart cells as its muscle cells can divide, making them an excellent model for cardiomyocyte repair.
They can also regenerate retinal neurons, restoring visual function, unlike mammals. Understanding the molecular underpinnings of this capability can advance research in human retinal regeneration and vision restoration. A 2022 study in the Journal of Neuroscience examined retinal regeneration in a zebrafish model of inherited retinal degeneration caused by the cep290 gene mutation. Single-cell RNA sequencing (scRNA-seq) revealed sustained expression of Notch3 and other quiescence genes in cep290 mutants, an observation not detected with bulk RNA-seq. This single-cell data was crucial for understanding the molecular basis of failed regeneration in this chronic disease model, highlighting Notch signaling as a key barrier to retinal cell regeneration. This underscores the power of single-cell approaches for dissecting complex tissue responses in disease states.
Similarly, the fruit fly (Drosophila melanogaster), known for its occasional escapes from the fly-room to the neighboring laboratories, has been the cornerstone of genetic research since the beginning of the 20th century.
Like zebrafish, fruit flies are small, have short reproduction cycles, and are cost-effective to maintain. They share 75% of disease-causing genes with humans. The Notch and Wnt signaling pathways, critical for supporting cellular and tissue development and adult homeostasis, are remarkably conserved between humans and fruit flies, making the latter vital for understanding human disease complexities, including reproductive disorders.
The mushroom body, Drosophila’s brain computational center, is a highly complex neural circuit for associative memory and sensory information processing. The principles of neuronal diversity and complexity in Drosophila have parallels in more complex organisms, including humans. A University of Oregon team used snRNA-seq to explore the diversity of cell types in the brain, focusing on neurons from T2 neuroblasts. They identified over 150 distinct cell clusters, mapped neurotransmitter and neuropeptide expression, and identified unique transcription factor combinations for each cluster. The research mapped the brain atlas with known neuron subtypes to specific clusters, such as olfactory projection, serotonergic, dopaminergic, octopaminergic, mushroom body neurons, supporting the hypothesis that each cluster represents one or a few closely related neuron classes.
This research provides a framework for understanding human brain complexity by elucidating how different neuron types are generated and connected. Understanding the genetic and molecular basis of neuron diversity and connectivity can identify potential therapeutic targets and enhance stem cell-based approaches for brain repair.

Larger Models
While the preference for smaller mammals or non-mammals is due primarily to their ease of use, larger animals have organ systems more similar in size and complexity to those of humans. They more accurately mimic disease progression and provide better information on drug efficacy and safety.
Chickens
Chicken embryos are a well-defined developmental model because they develop externally and are large enough to manipulate experimentally. They are accessible for procedures like implantation experiments, and provide insights into conserved developmental mechanisms across vertebrates.
To understand the effect of the FGF2 molecule on lens development, a team of researchers from Miami University at Oxford, OH, used snRNA-seq on the eye tissue of chicken embryos to profile gene expression in individual lens cells. They utilized a retina regeneration model to assess the effects of the FGF2. They found a decrease in epithelial cells and changes in intermediate and fiber cell states post FGF2 stimulation. The study also confirms the activation of MAF, a gene influenced by FGF, which is crucial for fiber cell differentiation. Additionally, the study suggests that the retina is a vital source of growth factors, supported by the finding that gene expression levels drop after retinectomy but are restored with FGF2 addition.
The discovery described in this work could pave the way for novel therapeutic approaches to human retinopathies.
Livestock
Research in livestock reproduction is critical, as it underpins the strength of a critical sector of national economies by bolstering food security and economic strength.
A team from the University of California, Davis, used scRNA-seq to provide insights into the effects of the NANOS3 gene knockout in cattle. NANOS3 is critical in germline development of both sexes, but it is not well characterized. In this long term, longitudinal study, the researchers used cells from 90 day fetal and 283 perinatal gonadal tissues and fixed them before use.
Compared to wild-type, the NANOS3 KO cattle had no germ cells at 283 days, altered gene expression, and possible impairment of somatic gonad development – due to lack of germ cells. These findings demonstrate that NANOS3 is necessary for both male and female fertility in cattle.
Non-Human Primates (NHP)
Non-human primates (NHPs) are essential for translational research because of their close genetic, physiological, and behavioral similarities to humans. They play a crucial role in the development of new therapies, vaccines, and medical devices. NHP models are often essential for preclinical studies to establish the potential efficacy of interventions before they can be tested in human clinical trials.
NHPs are used to study a wide range of significant human diseases, including HIV/AIDS, tuberculosis, hepatitis, neurodegenerative diseases, diabetes, cardiovascular diseases, respiratory diseases, and vision impairments.
For example, in a longitudinal study, scientists at the Tulane University School of Medicine analyzed the immune response of SIV-infected macaques during a SARS-CoV-2 coinfection. The team found that despite significant immunodeficiency, the SIV-infected macaques did not develop more severe COVID-19 symptoms than their non-SIV-infected counterparts. This challenges the narrative that HIV infection necessarily leads to worse COVID-19 outcomes, suggesting that other factors may be at play in the increased risk observed in some human studies.
The study also revealed aspects of the immune response in coinfected animals. The development of SARS-CoV-2-specific T cell and antibody responses was significantly impaired, a finding that aligns with observations in some humans. This impairment raises critical questions about vaccine efficacy and the risk of reinfection in immunocompromised individuals.
The researchers used scRNA-seq on bronchoalveolar lavage (BAL) cells collected before SARS-CoV-2 inoculation and on days 2, 7, 21, and 28 post-challenge. The researchers fixed the cells and stored them until all samples were collected. The possibility to fix the cells and their whole transcriptome enabled them to perform a comprehensive longitudinal analysis of the immune response in the lungs over the course of SARS-CoV-2 infection. This gave them a granular view of the immune response, highlighting the crucial role of innate immunity, particularly monocytes and macrophages, in controlling SARS-CoV-2 infection when adaptive immunity is compromised. This insight points to new therapeutic approaches for managing COVID-19 in immunocompromised individuals.