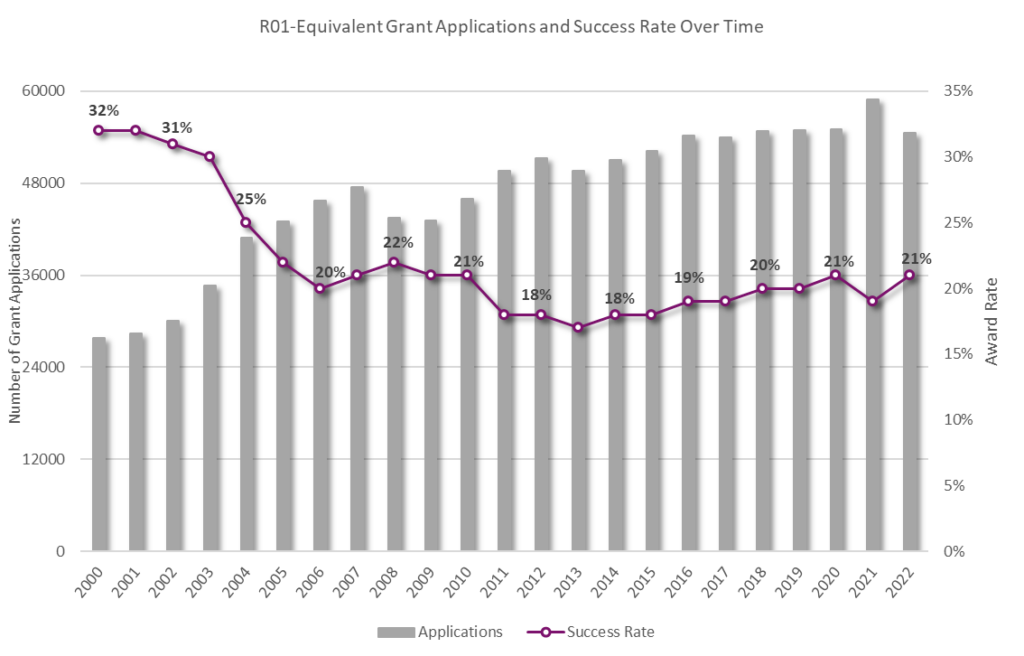
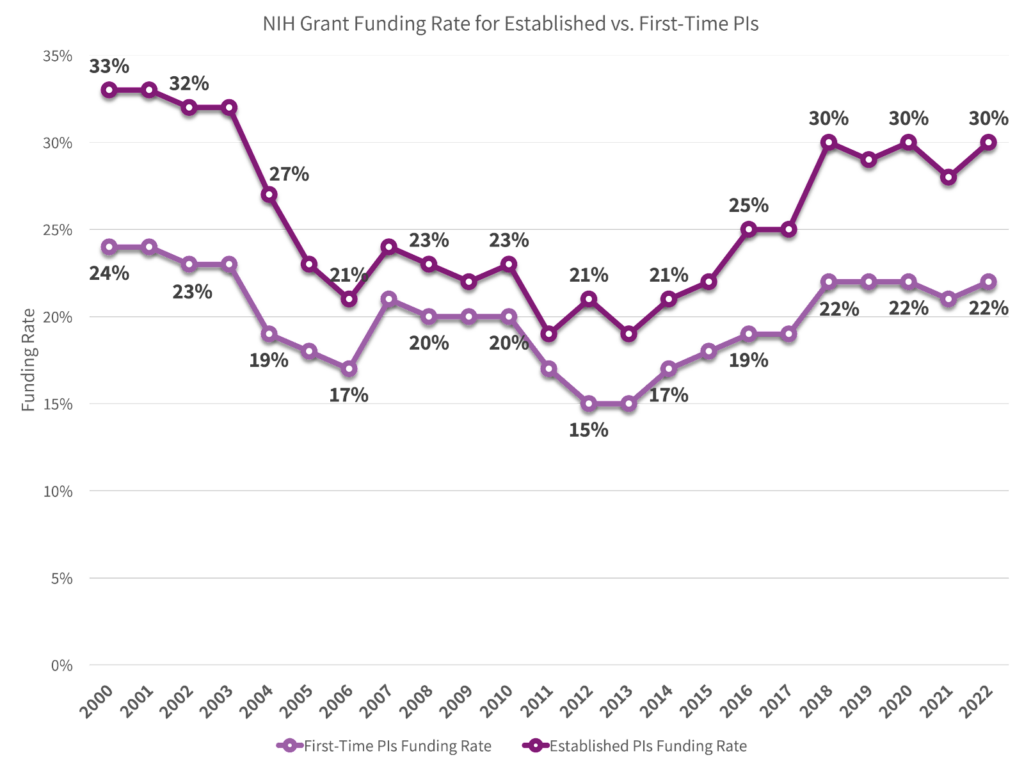
In the competitive grant submission arena, following best practices significantly improves the likelihood of funding.
Reviewers ensure fundamental aspects of a grant are present and convincing. A well-constructed grant application includes the following elements:
Research Significance
Research significance for the NIH and NSF have a similar meaning, addressing critical barriers to progress. It is a key factor in determining the impact score and, therefore, the chance for funding. Research significance emphasizes the project’s potential impact and addresses an important problem that aligns with the funding agency’s priorities.
The significance section of a grant provides reviewers with enough background to understand the work in its context. Robust applications address the knowledge gap and describe prior work’s strengths and weaknesses relevant to the research question.
The research significance section proposes how the researcher addresses gaps and advances scientific understanding.
Novelty
Reviewers consider the project’s potential for innovation. The innovation section of a grant proposal demonstrates the originality of the approach or model. For instance, does the work move the field forward by introducing novel concepts?
Substantiating the novelty claim involves providing references to previously identified gaps and clarifies the importance of applying a new approach.
There are three common reasons projects are considered innovative: the central hypothesis itself is new, the model system developed to test the hypothesis is unique, or the technical approach bridges two methodologies from different fields.
Scientific Approach
The scientific approach section describes the overall strategy to accomplish the project’s goals. The section discusses potential alternatives and anticipated benchmarks.
Reviewers look for a well-reasoned and appropriate plan, complete with a timeline for the completion of the project.
Here, the best practice is to use an approach based on preliminary results. While preliminary data are necessary for most grants, it may not be for small or developmental grants. When previous work is unavailable, provide a plan to acquire early data with following actions based on potential outcomes.
Include subsections to the section addressing each project’s aim and the expected outcomes.
Likewise, detail a plan describing the compilation and reporting of collected data, including experimental setup, sampling strategy, and data analysis. Including a high level of detail exhibits the integration of rigor and reproducibility in the experimental design. High scoring applications specifically identify and control for inherent biological variability to strengthen the research conclusions.
If the study includes research subjects, provide a plan to protect their rights and well-being. Secure approval from the Institutional Review Board (IRB) for the research protocol in advance, if prudent. Key points convincing the reviewers that ethical principles and guidelines are followed include:
- Description of the target population with inclusion and exclusion criteria.
- Detailed risks and benefits to the participants. Include how safety is monitored, and personal data is managed and protected.
- Specifics for obtaining participants’ consent and authorized personnel to initiate the conversation.
On this delicate matter, the NIH provides extensive guidance.
Infrastructure
The scientific environment where the work will be done must meet the project’s needs. For this reason, describe institutional support for equipment and resources to demonstrate the project is feasible and sustainable.
List and describe shared facilities available to the project, such as institutional genomics cores. These include any facilities or staff required to conduct experiments, the collection of samples, sample preparation, and data analysis.
Federal funding agencies also encourage collaborative arrangements with other research centers as long as the resources are available at each site.
Include a description of the policies in place to manage, share, and protect data integrity and ethics. The details are especially important if research subjects are involved.
Financial Discipline
A strong grant demonstrates a complete understanding of the research effort, including anticipated categories and expenses. The budget includes investigators’ time, equipment, supplies, and travel expenses.
The NIH recommends identifying necessary and reasonable costs as the reviewers judge the expenses against the research proposal. Over or under-estimating the costs implies an incomplete understanding of the project scope.
The NIH provides a helpful checklist of items to remember when creating a budget.