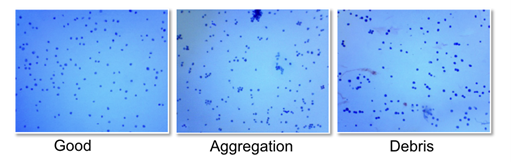
Designing a successful scRNA-seq experiment involves several key considerations, from sample selection and preparation to quality control. There are a few critical factors to consider when planning a scRNA-seq experiment to optimize the experiment while avoiding common pitfalls.
Sample Size
An important consideration that could even impact the choice of scRNA-seq technology is the sample size — researchers need to sequence enough cells to answer their biological question.
While microfluidic-based technologies may limit the ease of scalable sample size, combinatorial barcoding technologies don’t have that limitation as they are plate-based.
Experimental planning tools are ideal for making such decisions. Numerous tools like the Single Cell Experimental Planner enable scientists to plan experiments based on specific needs. Important points to consider are:
- What type of study — is it for a publication, a pilot, a small research project, a cell atlas, a drug screening?
- Number of cells needed/available
- Number of samples available
- Sample type
- Sequencing needs and suggested platforms
Sample Type
Before planning the experiment, a decision point is choosing between sequencing whole cells or just nuclei. Each approach has its pros and cons, and the decision depends on the research question and nature of the samples. Choosing nuclei sequencing is beneficial for cells difficult to dissociate without compromising viability.
For instance, highly fibrous tissues — brain, skin, tumors with a high amount of extracellular matrix — are challenging to dissociate, often resulting in cell death.
Moreover, some samples, like cells from three-dimensional cultures, present challenges. Once extracted from their three-dimensional matrix, these cells perish quickly.
For such scenarios, the alternative is single nuclei sequencing. Most genes reside in the nucleus, so sequencing nuclei captures nearly the same transcriptomic data despite a nominal loss of RNA from the cytosol.
In clinical contexts, nuclei are invaluable. They permit immediate freezing of tissue samples, whether sourced from the operating room or from sizable tissue harvests.
Opting for nuclei over whole cells can also facilitate extraction from samples stored in liquid nitrogen.
Moreover, the constraints of the chosen technology play a role in deciding between cells and nuclei. If a researcher deals with larger cells and the chosen technology restricts cell size, as seen with pico-wells or certain droplet-based systems, then nuclei become the inevitable choice.
Number of Samples
The number of samples analyzed plays a crucial role as it affects the accuracy of overall results and increases the power of statistical tests. One reason for manuscript rejection is the need for more technical or biological replicates within the study. The same holds true for studies that leverage scRNA-seq as a primary research method.
But if the sample size is inadequate, pooling becomes a viable solution. There might be a need for more cells for the assay, especially with embryonic samples, rare organisms, or when viable cells are scarce. In such cases, combining samples can address the issue.
For instance, pooling several samples from distinct model organisms (like drosophila embryos) or combining multiple sections of an identical tissue creates enough biological mass to meet minimum cell counts of snRNA-Seq sample preparation. Additionally, the ability to fix samples allows the accumulation of cells or nuclei over time, making pooling logistically more feasible.
Replication is another key aspect of good experimental design that involves the use of an adequate number of samples.
There is a difference between technical and biological replicates and their respective applications (Figure 1).
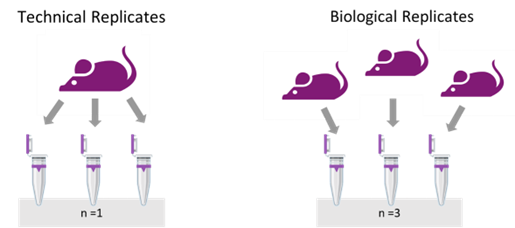
Figure 1: A well-thought experimental design considers technical and biological replicates to account for sources of variability.
A technical replicate measures the noise of the protocols or equipment by dividing the same sample into two or three sub-samples and processing them separately.
In contrast, biological replication entails examining biologically different samples under identical conditions — multiple subjects with the same disease or cells from different donors treated with the same stimulus. This approach captures the inherent variability in biological systems and verifies the experiment’s reproducibility.
Bioinformatic Support
A common concern for the novice scRNA-seq researcher is bioinformatics support. The computational aspects of single cell sequencing can be daunting. The good news is that there are now many user-friendly software options available that don’t require extensive programming knowledge, so that bench scientists now don’t have to double as computer scientists to obtain reliable and publication ready data.
It is also good practice consulting with bioinformaticians within the department or core facility during the planning phase of your experiment.
Fresh or Fixed?
One last critical decision is the use of fresh or fixed samples.
When running an experiment, researchers are focused on capturing a snapshot in biology, whether it be a specific developmental stage, a series of individual time points within a time course experiment.
Cellular metabolism and gene expression rapidly change once cells are removed from their physiological environment. Consequently, delays in sample processing lead to results that reflect stress responses rather than true biological states. Therefore, minimizing processing time is crucial in scRNA-seq experiments.
Fixation addresses the issue as researchers can dissociate the tissue, fix it, and store it.
Storing fixed samples in the freezer for later use streamlines many experimental logistics and lab research situations, such as:
Clinical Laboratory Setting
Working with fresh samples in a clinical laboratory presents several logistical challenges. For instance, tissues arriving from the operating room can do so at unpredictable times, disrupting experiment schedules and staffing. Even with lab prepared tissues, where timing is known, there’s the added task of booking instruments and arranging transport to the core facility.
Large scale projects
Large-scale projects can be particularly demanding in scRNA-seq. Time course experiments require sequential sample collection over long periods. Processing fresh samples for each time point can lead to batch effects that obscure the study variables. With fixation, this is no longer an obstacle.
Experiment setup time is another significant concern. Sample preparation from tissues and organs is labor-intensive, maintaining cell viability throughout the process is crucial, requiring careful coordination among lab members and efficient resource allocation. Planning sample accrual and fixation puts researchers back in charge of their own experiments.
Technical Variability
Another critical aspect is managing technical variability. Samples collected over extended periods are susceptible to batch effects due to inconsistencies in collection times, environmental factors, and even technician performance. These variabilities, often hard to control, can significantly impact experimental results.
Using a plate-based combinatorial barcoding method can be a valuable solution, as the user can fix, store, and later run up to 96 samples with a single kit.
Fixing cells or nuclei post-preparation offers flexibility to conduct experiments at a more convenient time (Table 1).
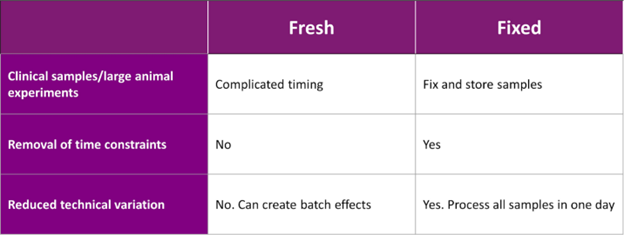
Table 1: Common considerations of scRNA-Seq experimental design: comparison of fixed and freshly prepared cells.